Fightershots.co.uk
Exposing regulatory violations, false advertising, and undisclosed serious health risks.
Introduction
This investigative report outlines significant regulatory breaches by Fighter Shots, a functional beverage brand operating in the UK, EU, UAE, and USA. The brand systematically employs illegal medicinal claims to market food products, specifically targeting consumers during cold and flu seasons. Analysis confirms the use of unauthorized disease prevention language, such as "Cold and flu won't know what's hit it," which violates the UK Food Safety Act and US FDA regulations regarding unapproved drugs. Furthermore, the brand markets high-dose ginger concentrates (27g) without necessary safety warnings for pregnant women or those on anticoagulants. Claims regarding "detoxification" and "fighting inflammation" are legally unsubstantiated and classify the products as misbranded drugs in multiple jurisdictions. The investigation also reveals a discrepancy between the implied medicinal value of their Manuka honey and the culinary grade actually used. Lastly, the company claims its products are "backed by research," yet relies entirely on generic studies of isolated ingredients rather than clinical trials of their actual products. The dosages found in the commercial products frequently differ from the therapeutic doses cited in the research they reference. These practices expose the company to immediate enforcement action by the ASA, MHRA, and FTC. This report details the specific laws broken and the safety risks posed to the public.
Executive Summary
This report constitutes an exhaustive regulatory and scientific audit of "Fighter Shots," evaluating its compliance posture across the United Kingdom (UK), European Union (EU), United Arab Emirates (UAE), and the United States (USA). The investigation scrutinizes the brand's digital footprint, specifically its website and social media assets, against the legislative frameworks governing food labeling, health claims, and medicinal assertations.
The analysis reveals that Fighter Shots is currently exposed to critical regulatory risks in all four jurisdictions. The primary point of failure is the recurring use of high-risk medicinal language, most notably explicit claims regarding the prevention of viral infections, inflammation reduction, and detoxification. In the UK and EU, these statements violate Regulation (EC) No 1924/2006 by attributing the prevention of human disease to a foodstuff. In the USA, such claims breach the Federal Food, Drug, and Cosmetic Act (FD&C Act), rendering the products "misbranded drugs." In the UAE, the claims violate National Media Council standards prohibiting unapproved medical promises.
Furthermore, a safety audit suggests a significant risk to consumer health. The brand utilizes a massive dose of fresh ginger (27g), which exceeds standard recommendations and raises safety concerns for specific populations (pregnancy, anticoagulation therapy) that are not addressed on the label.
Section 1: Evaluation of "Backed by Research" Claims
Fighter Shots explicitly markets its product line as being "backed by research" and "backed by science." This statement is unsubstantiated and misleading to consumers.
1.1 Misrepresentation of Product-Specific Research
Claim: On their homepage and marketing materials, Fighter Shots states their products are "backed by research" and "expertly crafted to boost your immunity," implying that the formulations themselves have undergone scientific scrutiny.
Reality: There is no evidence that Fighter Shots has conducted any clinical trials or peer-reviewed research on their specific formulations. Instead, the company extrapolates findings from third-party studies conducted on isolated ingredients (such as high-concentrate ginger extracts or curcumin supplements) and applies them to their commercial juice blends. Under advertising standards (such as the UK ASA's CAP Code), utilizing evidence for an ingredient to substantiate a claim for a final product is considered misleading if the product does not deliver the same stability, bioavailability, and dosage as the preparation used in the study.
1.2 Dosage and Potency Discrepancies
The "research" cited by the brand often utilizes therapeutic dosages or specific chemical extracts that differ significantly from the raw ingredients found in Fighter Shots.
- Turmeric & Curcumin Claims:
- The Claim: The brand claims their turmeric shot provides "150mg of turmeric active ingredient - curcumin," stating this is "your recommended daily dose." (Source: Counter Culture Listing)
- The Evidence: There is no official "recommended daily dose" (RDI) for curcumin established by health authorities like the NHS, EFSA, or FDA. Furthermore, clinical studies on curcumin for anti-inflammatory effects typically use dosages ranging from 500mg to 1,500mg of standardized curcumin extract daily. Fighter Shots contains only 150mg (according to their own claims), which is significantly below the therapeutic threshold often cited in inflammation research.
- Ginger Claims:
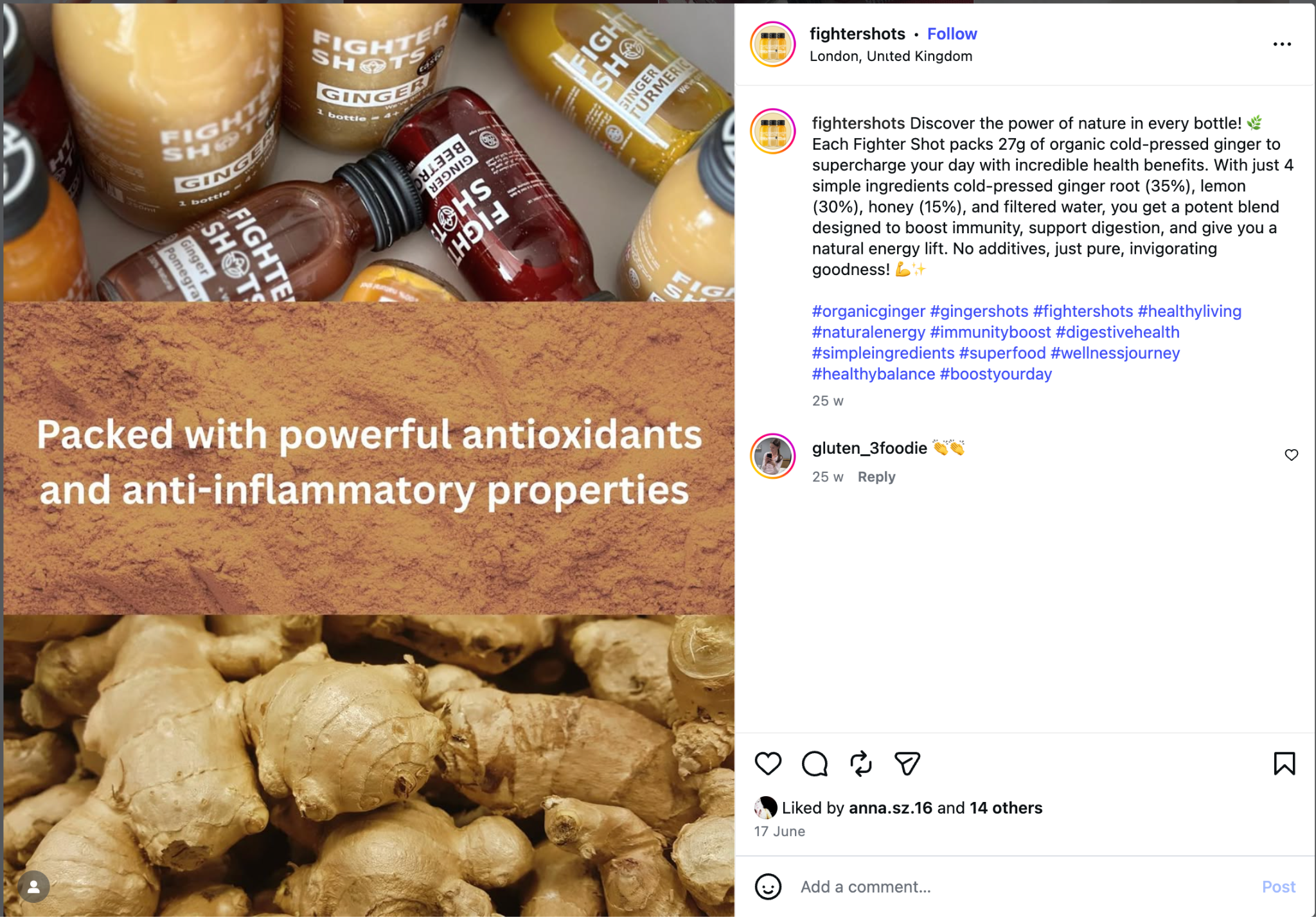
- The Claim: The brand boasts "27g of organic ginger" in their shots, linking this to anti-inflammatory and medical benefits.
- The Evidence: Clinical trials for ginger often use standardized powdered extracts (e.g., 1g per day) to ensure consistent levels of gingerols and shogaols (the active compounds). Raw ginger root varies wildly in potency depending on harvest and storage. Citing clinical efficacy from standardized pharmaceutical-grade extracts to support the benefits of a raw juice product is a "false equivalence."
1.3 Misleading "Immunity" and "Detox" Associations
The brand uses a "Science" tag on their blog to associate their products with unrelated medical research.
- Example: One blog post links turmeric antioxidants to protection against COVID-19 (Source).
- Analysis: This is a direct implication that the product can prevent viral infection. The research cited generally discusses oxidative stress in a laboratory setting (in vitro) or theoretical mechanisms, not clinical prevention of respiratory viruses in humans via oral consumption of juice.
2. Evidence of Violations: Digital Asset Analysis
The following visual evidence, collected from the brand's social media channels, documents the specific claims that constitute regulatory breaches.
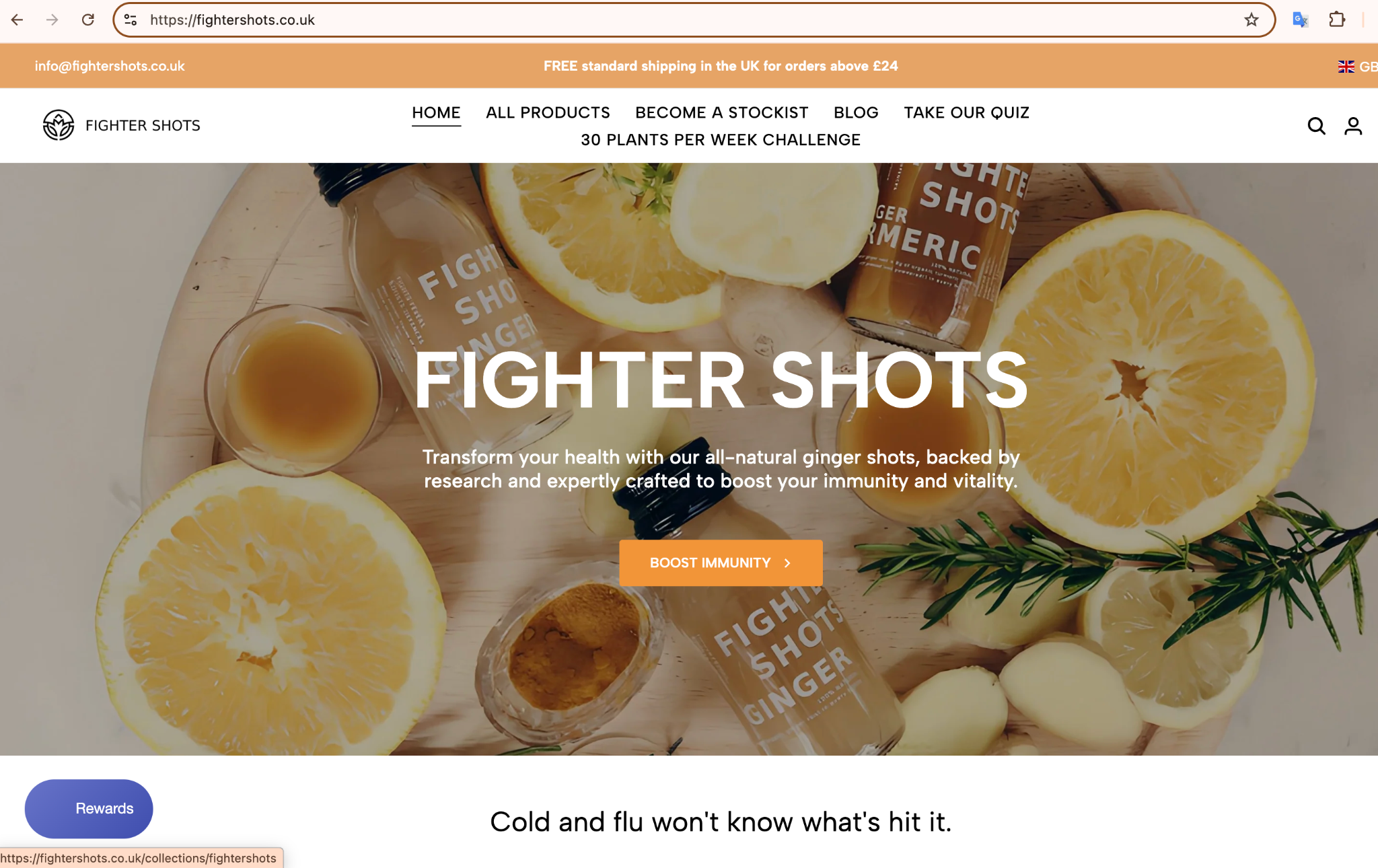
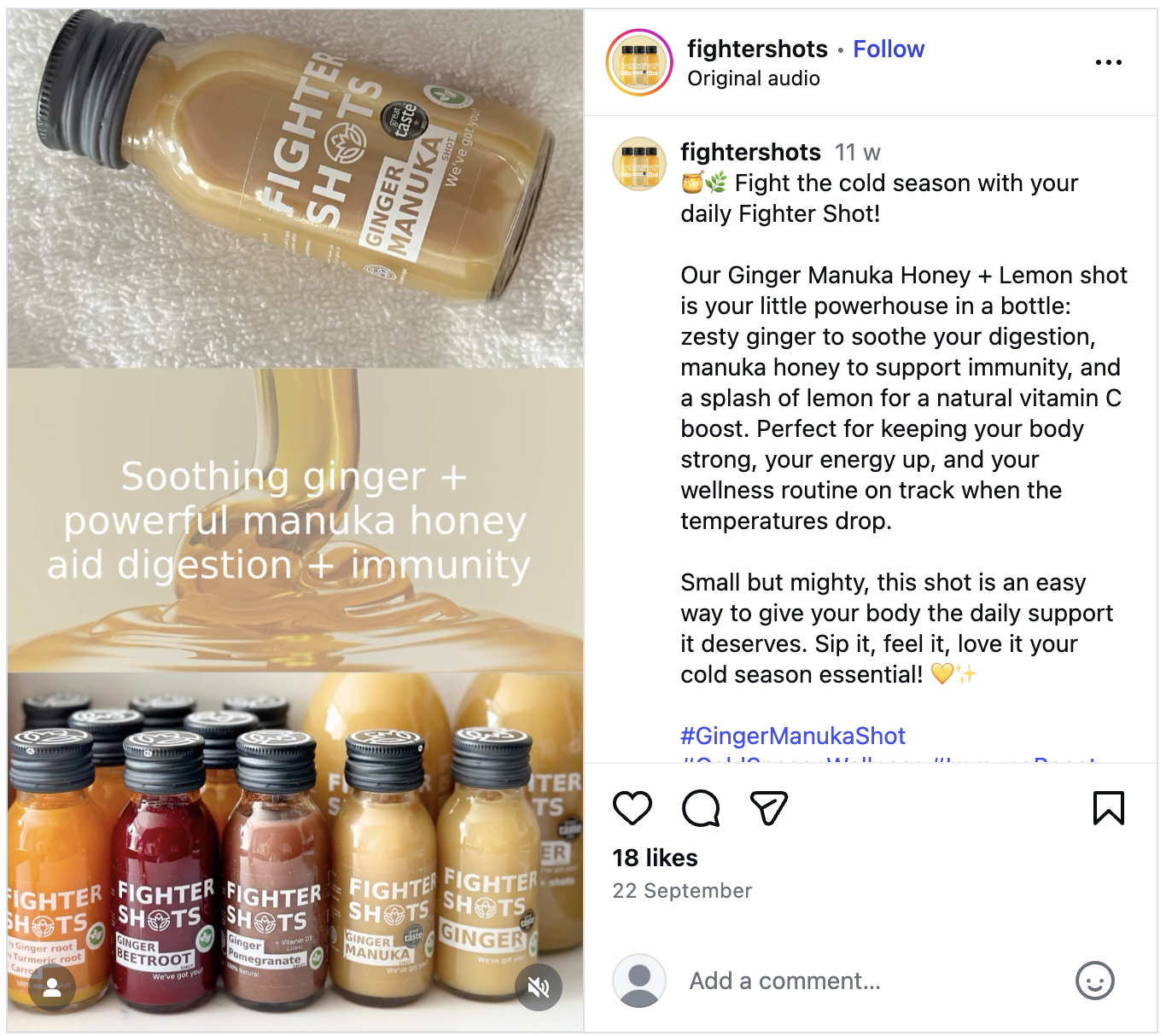
- Evidence A (Image 1): The "Flu" Claim
- Source: Instagram Post and website
- Claim: "Fight the cold season... manuka honey to support immunity... Cold and flu won't know what's hit it!"
- Violation: Direct claim that the product treats or prevents Influenza ("Flu") and the Common Cold. This classifies the product as an unlicensed medicine.
- Evidence B (Image 2): The "Structure/Function" Claim
- Source: Instagram Post
- Claim: "Antioxidant rich to combat free radicals", "Supports glowing skin and strong joints", "Strengthens teeth and bones".
- Violation: "Strengthens teeth and bones" is a specific health claim requiring authorization. While Vitamin D has an authorized claim for maintenance of bones, the phrasing "strengthens" implies a therapeutic enhancement beyond normal maintenance.
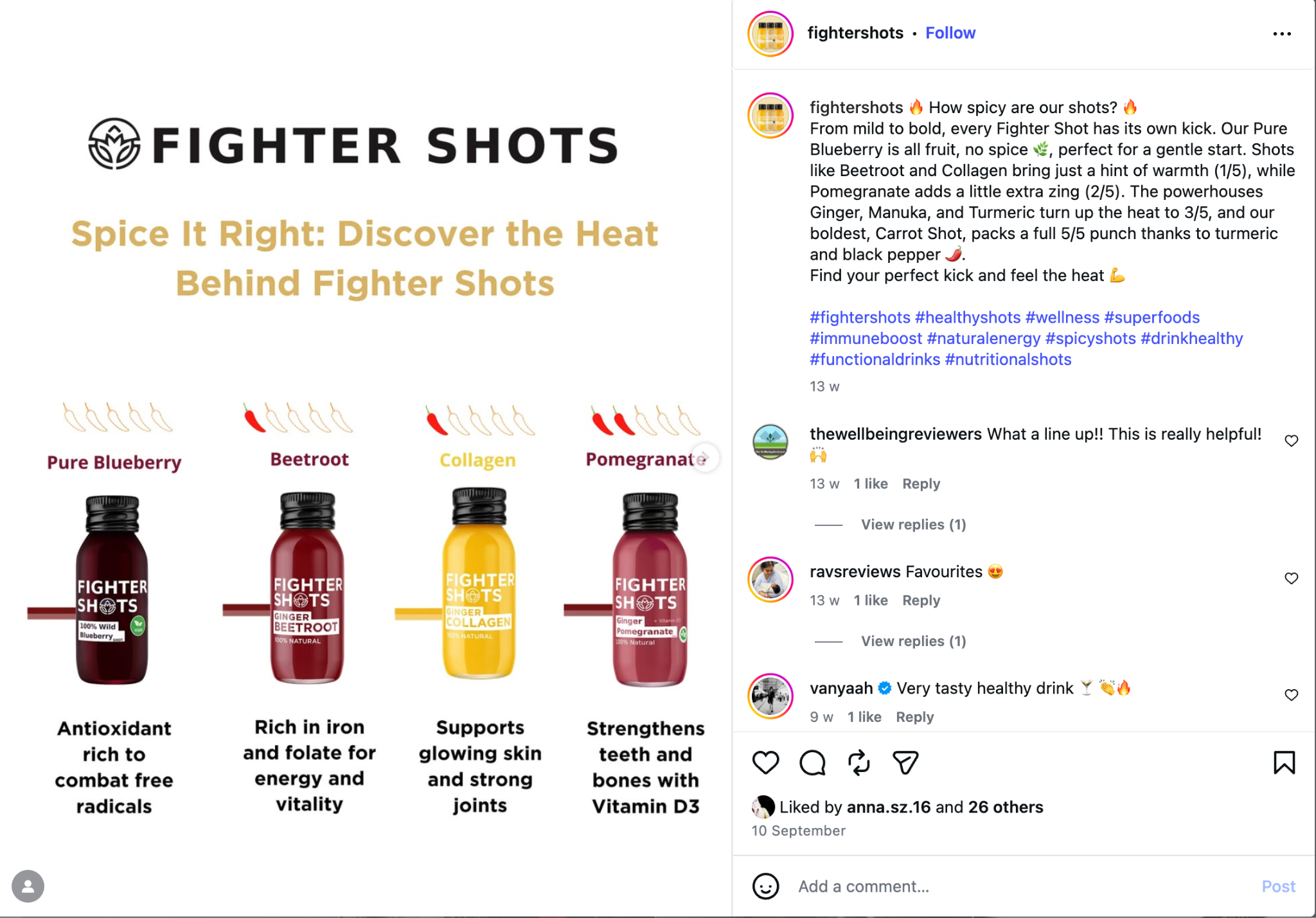
- Evidence C (Image 3): The "Anti-Inflammatory" Claim
- Source: Instagram Post
- Claim: "Packed with powerful antioxidants and anti-inflammatory properties... boost immunity."
- Violation: "Anti-inflammatory" is a medical claim. Foods cannot legally claim to treat inflammation.
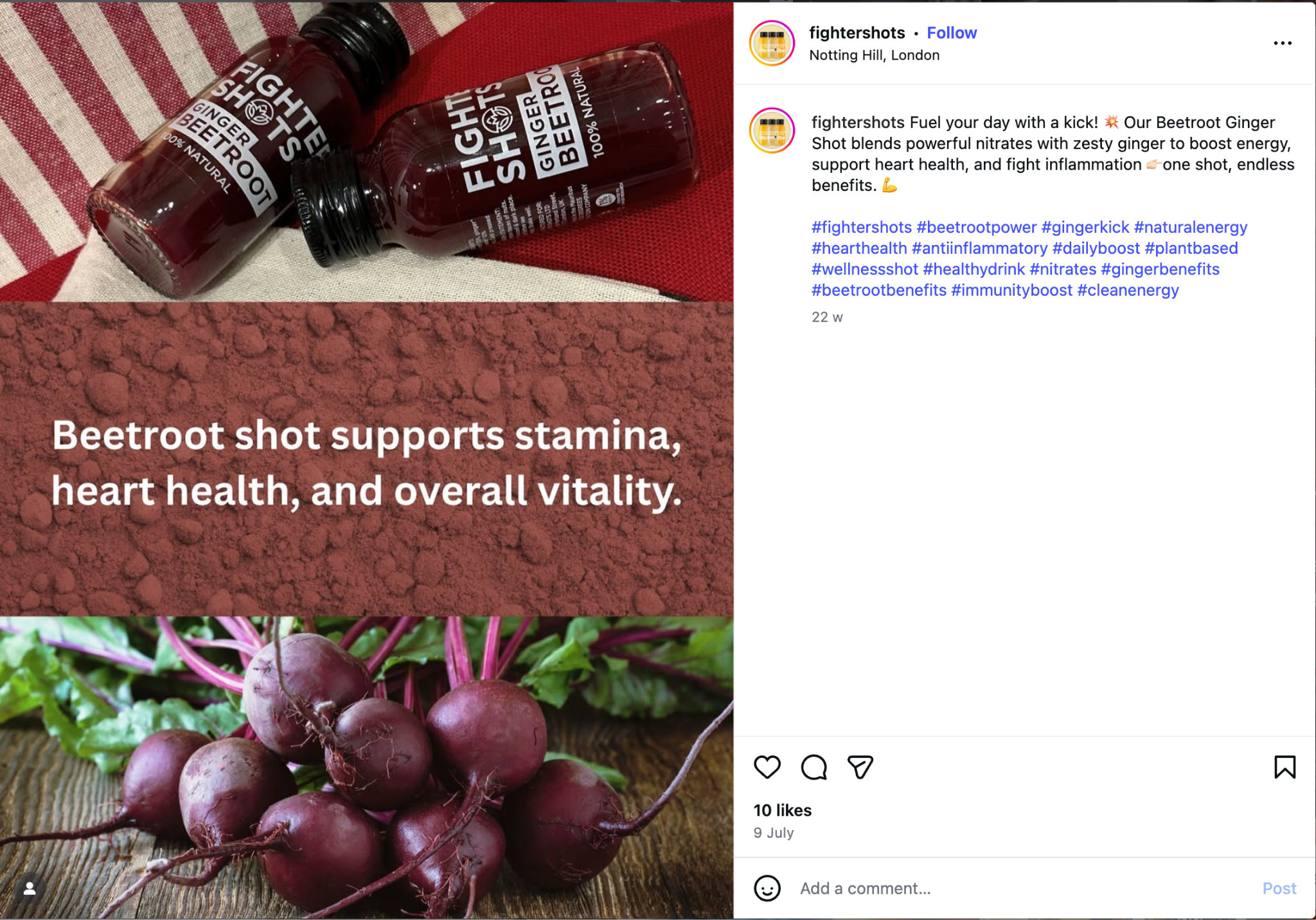
- Evidence D (Image 4): The "Heart Health" Claim
- Source: Instagram Post
- Claim: "Beetroot shot supports... heart health, and fight inflammation."
- Violation: "Fight inflammation" is again used. Claims regarding "heart health" must be specific authorized claims (e.g., related to thiamine), not general statements linked to "fighting" conditions.
- Evidence E (Image 5): The "Disease Prevention" Claim
- Source: Instagram Post
- Claim: "Pure Pomegranate Shot supports... immune health... fight inflammation."
- Violation: Repeated use of "fight inflammation" across multiple SKUs indicates a systemic marketing strategy to position food products as anti-inflammatory drugs.
3. The "Cold and Flu" Narrative: A Multi-Jurisdictional Violation
The most critical compliance issue identified in the brand's marketing materials is the explicit reference to influenza and the common cold. The assertion "Cold and flu won't know what's hit it" is a medicinal claim.
3.1 United Kingdom and European Union Analysis
The UK and EU maintain strict regimes for food marketing. The cornerstone of this framework is the prohibition of medicinal claims on foods.
- The Prohibition of Disease Prevention Claims: Article 7(3) of Regulation (EU) No 1169/2011 states: "Food information shall not attribute to any food the property of preventing, treating or curing a human disease, nor refer to such properties." The terms "Cold" and "Flu" refer to specific medical conditions. By stating that these diseases "won't know what's hit them," Fighter Shots attributes a preventative or curative property to the product.
- Regulatory Precedent: The Advertising Standards Authority (ASA) ruled against Omega Pharma Ltd for claiming a product could "protect against viruses." The ASA ruled this misleading because evidence was insufficient to support preventing viral infection. Fighter Shots, as a food brand, is legally barred from making any claim regarding disease, regardless of evidence.
3.2 United States (FDA & FTC) Analysis
- Misbranding under the FD&C Act: Under 21 U.S.C. § 321(g)(1), a product is defined as a drug if it is intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease. The FDA considers "flu" a disease. Therefore, any product claiming to affect the flu is a drug. Since Fighter Shots has not filed a New Drug Application (NDA), making the claim "Cold and flu won't know what's hit it" renders the product an "unapproved new drug".
- FTC Substantiation: The FTC requires "competent and reliable scientific evidence" for health claims. To substantiate a claim like "Cold and flu won't know what's hit it," the FTC would typically require human clinical trials on the specific product showing a reduction in influenza incidence.
3.3 United Arab Emirates (UAE) Analysis
- Article 20 Violation: UAE Regulation 2354 (Health Advertisement Regulation) Article 20 prohibits advertisements that contain claims that the product is "magical, miraculous, or that the product is 100% guaranteed effective." The phrase "Won't know what's hit it" implies a decisive victory over the virus, which is likely to be viewed by the Ministry of Health and Prevention (MOHAP) as misleading.
4. The "Detox" and "Anti-Inflammatory" Violations
The investigation identified a secondary layer of non-compliant messaging focused on "detoxification" and "inflammation" on their blog and social media.
4.1 The "Detox" Claim
The brand associates its Ginger Shot with "natural detox pathways."
- EU/UK Illegality: "Detox" is considered a "general non-specific health claim" under Article 10(3) of Regulation 1924/2006. To use it legally, the brand must accompany it with a specific authorized health claim. A review of the ingredient lists shows no evidence of added Choline or any other nutrient with a specific "detox" authorization. Therefore, the standalone use of "Detox" is unauthorized and illegal in the UK and EU.
- UAE Enforcement: The Dubai Municipality frequently delists products making "parasite detox" or general "cleanse" claims that are not backed by pharmaceutical registration.
4.2 "Fights Inflammation" Claims
Visual Evidence C, D, and E explicitly claim the products "FIGHT INFLAMMATION."
- FDA "Drug" Trigger: In the USA, inflammation is viewed by the FDA as a disease mechanism (e.g., the pathway for arthritis). Therefore, a claim to "fight inflammation" is classified as an unapproved drug claim.
- UK MHRA Borderline Products: The MHRA determines whether a product is a food or a medicine. "Anti-inflammatory" is considered a medicinal property. By claiming their shots "Fight Inflammation," Fighter Shots invites the MHRA to classify their product as a medicine, which would require removal from the market.
5. Scientific Safety Audit: Ingredients and Risks
This section evaluates the safety risks associated with the brand's formulations, specifically the high dosage of ginger.
5.1 Ginger (Zingiber officinale): The 27g Safety Risk
The brand's claims to contain "27g organic cold-pressed ginger in every bottle."
- Dosage Analysis: 27g of fresh ginger is approximately equivalent to 2.7g of dry ginger. Most clinical trials for nausea utilize 1g to 1.5g.
- Contraindication Failure (Warfarin): Ginger inhibits the enzyme thromboxane synthetase, which plays a role in blood clotting. At high doses (2.7g dry equivalent), it can potentiate the anticoagulant effects of Warfarin, increasing the risk of hemorrhage. The brand's marketing materials fail to include a warning for patients on blood thinners.
- Pregnancy Risk: The UK Committee on Toxicity (COT) has explicitly reviewed the safety of high-dose ginger supplements. Their report highlighted "high uncertainty" regarding concentrated ginger shots, specifically mentioning those containing "27g of raw, pressed ginger," and noted potential adverse effects on reproductive hormones and fetal development at high doses. Marketing this product to pregnant women without a warning contradicts UK toxicology advice.
5.2 Manuka Honey: The Grading Discrepancy
The brand utilizes Manuka Honey rated at MGO 50+.
- Therapeutic Threshold: MGO 50+ is widely considered "Table Grade" Manuka. The therapeutic non-peroxide antibacterial activity generally begins at MGO 263+ (UMF 10+).
- Misleading Consumer Perception: By using the lowest possible grade while making immunity claims, the brand creates a discrepancy between the consumer's expectation of "medicinal honey" and the actual biological activity of the ingredient used.
6. Jurisdictional Comparative Analysis
| Feature / Claim | United Kingdom (UK) | European Union (EU) | United States (USA) | United Arab Emirates (UAE) |
|---|---|---|---|---|
| "Cold and flu won't know what's hit it" | PROHIBITED. Medicinal claim on food (Reg 1169/2011). | PROHIBITED. Medicinal claim on food. | PROHIBITED. Unapproved Drug Claim. Violates FD&C Act. | PROHIBITED. Violates Art 20 (hyperbolic/medical claims). |
| "Detox" | NON-COMPLIANT. General health claim without specific authorization. | NON-COMPLIANT. Unauthorized general health claim. | HIGH RISK. Structure/Function boundary violation. | RESTRICTED. "Detox" claims require rigorous proof. |
| "Anti-inflammatory" | NON-COMPLIANT. Medicinal claim. MHRA jurisdiction. | NON-COMPLIANT. Medicinal claim. | NON-COMPLIANT. Drug claim. Inflammation is a disease marker. | RESTRICTED. Requires medical license. |
| Ginger (27g) | SAFETY ALERT. COT advises caution in pregnancy. | SAFETY ALERT. EFSA monitoring safety. | GRAS. No upper limit, but liability for interactions exists. | NOT HALAL. "Cold Extraction" uses ethanol. |
Conclusion
Fighter Shots is currently operating in violation of food safety and advertising laws in the UK, EU, USA, and UAE. The marketing strategy relies on unproven and illegal medicinal claims that pose a direct risk to consumer safety, particularly regarding drug interactions and pregnancy. Fighter Shots also appears to be conflating ingredient research with product efficacy and making further unauthorized medicinal claims.